About the project
Objective
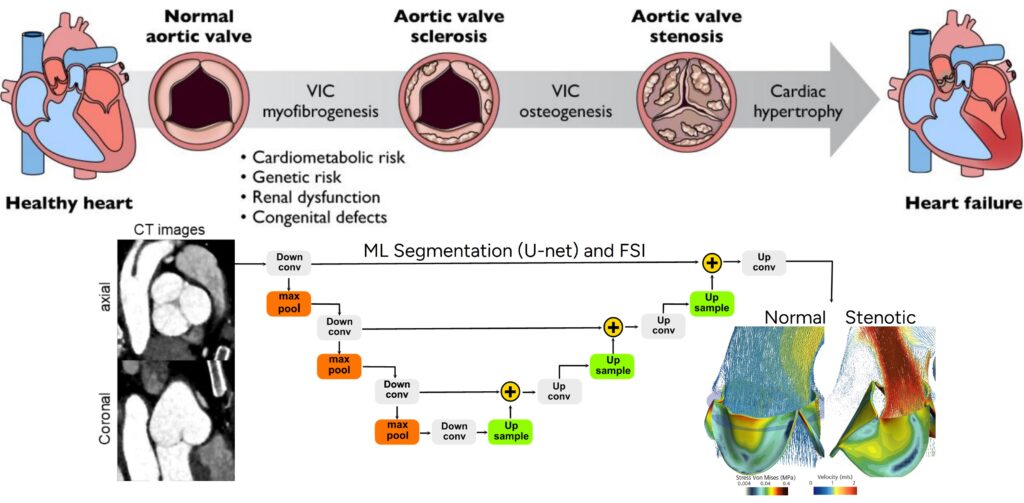
The overall objective of this project is to develop a predictive model for the progression of Aortic Valve Stenosis (AVS) by integrating repeated CT imaging, fluid–structure interaction (FSI) simulations, and deep machine learning techniques. Through comprehensive analysis of large-scale clinical datasets and high-fidelity simulations, this project seeks to move beyond conventional imaging and hemodynamic metrics to enable early detection, individualized prognosis, and improved clinical decision-making in AVS management.
Background
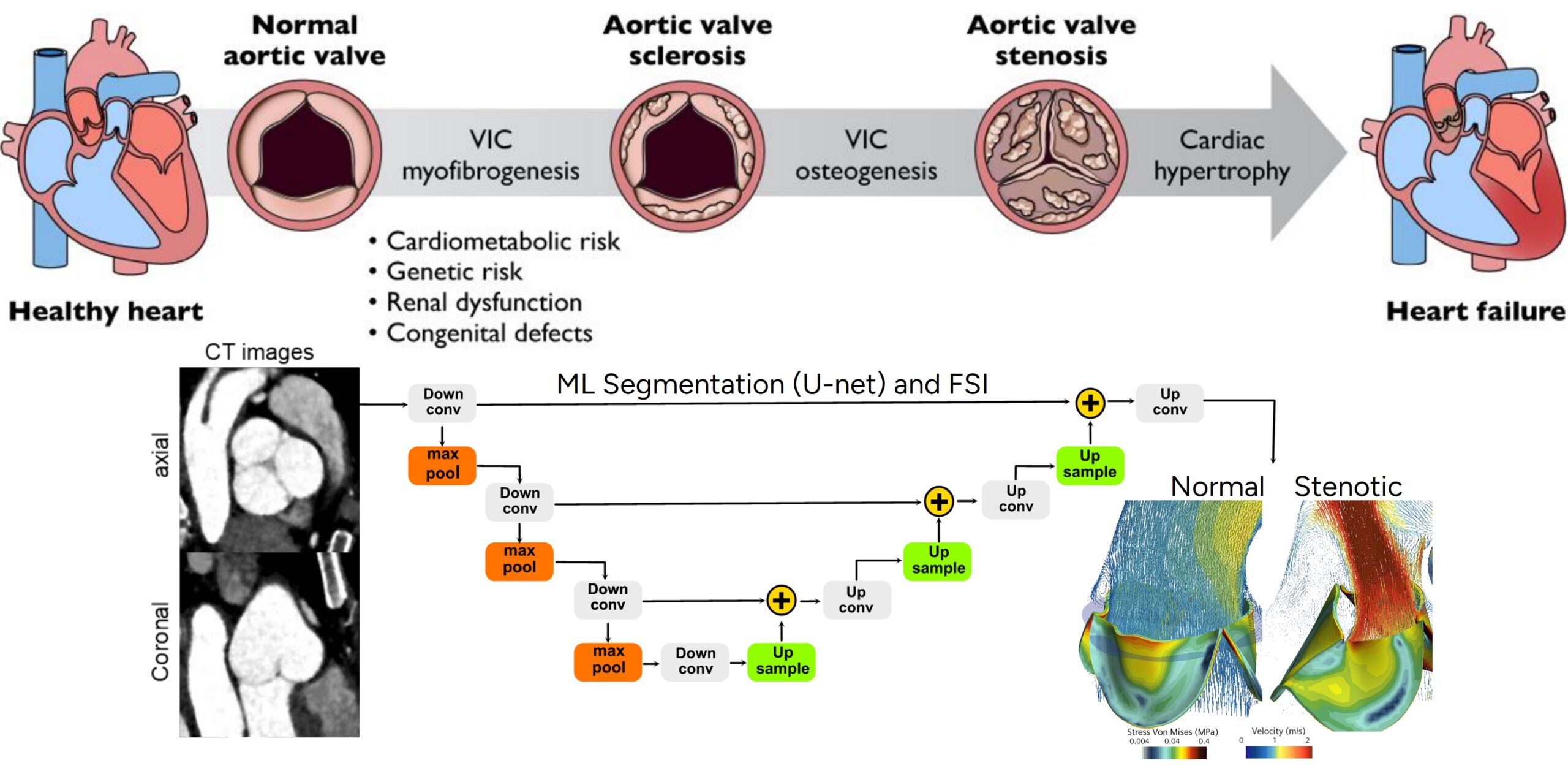
Aortic valve stenosis (AVS) is dangerous because it leads to obstructed blood flow from the heart, causing increased pressure within the heart and raising the risk of heart failure, arrhythmias, and other severe complications. Over 2 million people worldwide are affected by the condition, with its prevalence increasing as the population ages, and it is often diagnosed through imaging techniques such as echocardiography and CT scans. Treatment options include surgical and transcatheter aortic valve replacement (SAVR/TAVR), both aiming to relieve symptoms and improve heart function, though there is uncertainty in selecting the best approach for individual patients based on their health condition and the severity of the disease.
Therefore, there is a critical need for a physics-based understanding of the progression of AVS, as current recommendations of AVS management and monitoring do not fully account for the complex interaction of hemodynamics (blood flow) and biomechanics (tissue deformation), which drive valve degeneration and calcification. In particular, oscillatory shear index and high shear stress in certain regions of the valve and aorta, along with altered cyclic loading, contribute to the pathological changes in the valve, exacerbating stenosis and complicating treatment outcomes.
Cross-disciplinary collaboration
This project brings together a new cross-disciplinary and complementary collaboration uniting experts in cardiovascular imaging, fluid dynamics, and machine learning to advance predictive modeling of aortic valve disease:
- Elias Sundström, a researcher in Fluid Mechanics at the Department of Engineering Mechanics, KTH, specializes in developing computational models to elucidate the complex fluid–structure interactions that occur within the human body.
- Magnus Bäck, is a senior physician and Professor in Cardiology at Karolinska Institutet, Translational cardiology. Research focus on Aortic Valve Stenosis.
- Payam Esfahani, is a postdoctoral researcher at KI in Magnus Bäck’s research group. Research focus on using advanced AI/ML applied to biomedical imaging and will remain a key contributor to the development of the project’s methodologies.